European Authorised Representative
An Authorized Representative is either a person or an entity who is responsible for placing the medical devices of a non EU manufacturer in EU market under his own name, does all the communication with the notified body and register his devices with competent authority.
European representative should be chosen with care as is responsible for activities like product registration, products recall, vigilance, market surveillance etc. He also monitors the various post-marketing and vigilance issues related to product and are the contact person on your behalf who is contacted by European authorities in case of emergencies.
We have legal offices in Netherlands and UK for providing this service.
Requirements to be an Authorised Representative
The European Authorized Representative beholds a crucial responsibility in the domain of regulatory services for medical devices. Such a position requires careful introspection and an array of knowledge of the rules and regulations.
There are 3 primary requirements to become an EU Authorized Representative of any company:
- You must be a natural or legal person established in the European Economic Area (EEA), European Union (EU) & European Free Trade Association (EFTA).
- You should be explicitly designated (authorized) by a non-European manufacturer with a written mandate.
- And most importantly you should accept to be the representative of the respective company.
This seems easy but, before accepting this position, one must understand that an EU EAR has to comply with a lot of rules. After this designation, the EAR will be legally considered as the main representative for the manufacturer in Europe. So, if the manufacturer acts fraudulently, his EU EAR will be held responsible if the latter does not alert the Competent Authorities.
Also, having some prior work experience in the medical device quality and regulation field is generally recommended. This reflects more credibility because manufacturers generally don’t prefer a newbie. The reputation is very well taken into consideration while appointing an EAR making it important to keep learning and staying up-to-date with the regulations.
Points to be Taken Into Consideration while Choosing your European Authorised Representative

We at Meddevices, provide EU Authorised Representative Services through our office in The Netherlands.
Distributor as EU Authorized Representative
Some companies use their distributor or importer as their Authorized Representative. However, a distributor might not have a very clear understanding of all the roles and responsibilities of an Authorized Representative. In addition to this, one must also consider the following complications that arise when appointing your distributor as your EAR:
- As the name and address of your distributor acting as your Authorized Representative has to be mentioned on all your documentation including the labeling and the user manual, the manufacturer cannot easily take the decision of changing that particular distributor. This is because, a decision like this will lead to re-printing and re-doing of all the labels, instruction manuals and packaging, which is a very costly affair. Also, the manufacturer will have to find out a way to deal with the products that are already present in the market with that distributor’s name on them.
- Choosing one distributor as your Authorized Representative among several other distributors could potentially create a hierarchical situation amongst distributors. Those not appointed as Authorized Representative might feel less significant which may lead to animosity between the manufacturer and the distributors, ultimately leading to a negative impact on the product sales.
- The focus of the distributor is generally on the sales and marketing of the medical devices rather than on the Regulatory Affairs. In case, the European laws and guidelines are modified, the distributor may not always be well informed about the new changes, thus misleading the manufacturer on how these changes will affect his medical device. Ignorance in Regulatory Affairs can be very risky.
- When your distributor is your Authorized Representative, he will have access to the Technical File which may also include proprietary information.
- Another reason not to appoint your distributor as your EAR is that, in case the Competent Authorities question an incident or non-compliance that occurred with your product, the distributor might protect his interests first rather than defending your company.
Therefore, it is advisable not to appoint a distributor as your European Authorized Representative. But regardless of this decision of appointing your distributor as your authorized representative, it is essential that your EAR has some history and experience in this business and is well versed and up-to-date with the Rules and Regulations of the European Union.
Can You be your own EU Authorized Representative?
Yes!!
You can be your own Authorized Representative.
For example: If your company is based in China and opens an office in Germany. The German office can be your Authorized Representative. But this scenario will be possible with only one condition that is you need to have a Regulatory Compliance person at this office. This is because the objective is not only to have a European address but also to perform all the tasks of an Authorized Representative.
Also, after the changes between MDD and MDR, it is now important to have PRRC within your European Authorized Representative. Usually, EU Authorized Representative being a small structure, can hire a consultant to be the PRRC for your organization.
Can Your Authorised Representative get Audited?
Yes!!
Since the Authorized Representative is equal to the manufacturer for the Competent Authorities, therefore, an audit is something they should be ready for.
Also, all mandatory documentation should be available at the headquarters of the Authorized Representative. This step is important as the documents have to be presented to the Competent Authorities upon request.
A good document retention procedure is equally important. The manufacturer must ask his Authorized Representative to show the documents so as to ensure that the latter has the right version. The Authorized Representative must be ready for the audit at any given time.
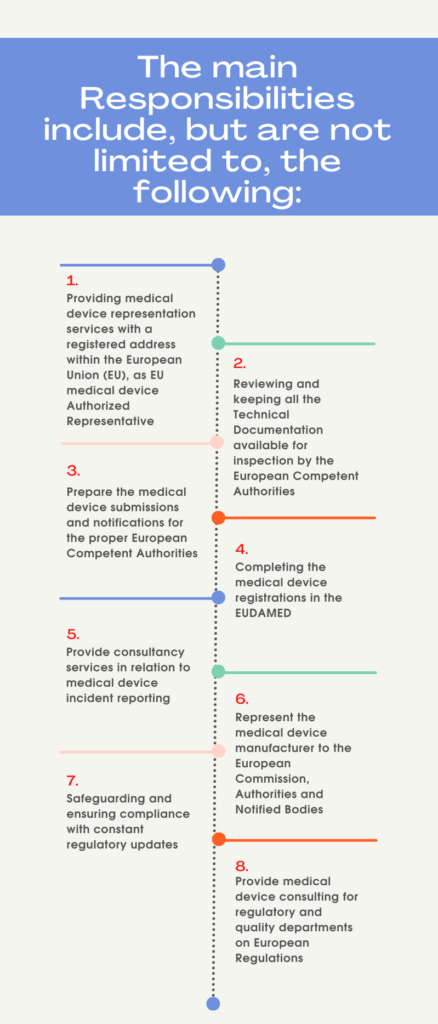
Roles and Responsibilities of an EAR
Medical Device Regulation – The new MDR EU 2017/745
The new Medical Device Regulation comes into effect on 26th May 2021- commonly referred to as “date of application”. Similarly, the date of application for the new IVDR is 26th May 2022. The MDR re-classifies certain medical devices and has a wider scope than the earlier MDD. It introduces an additional pre-market consultation procedure for certain high-risk medical devices. For IVDs, the biggest change concerns the risk classification of in-vitro diagnostic devices and the role of Notified Bodies. As a result, around 85% of all IVDs will need oversight from Notified Bodies, compared to 20% under the Directive.
There is a new clinical evaluation consultation procedure for Class III implantable devices and certain Class IIb devices, to be carried out by an independent expert panel. The Notified Body will have to take into consideration the scientific opinion expressed by the expert panel.
Unique Device Indicator – UDI
The new MDR article 24 introduces a completely new feature of Unique Device Identifiers (UDIs). The UDI will allow all stakeholders to access basic information on devices through the European Database for Medical Devices (EUDAMED). Each MD or IVD and, as applicable, each package will have a UDI composed of two parts. The first part is a device identifier (UDI-DI), specific to a manufacturer and a device. The second part is a production identifier (UDI-PI) – such as a lot number or a serial number, to identify the unit of device production and, if applicable, the package. Every level of packaging will be uniquely identified. For both Regulations, the deadline for assigning UDIs is the respective DoA. However, the obligation to affix the UDI on the labelling will be implemented in three stages.
