Introduction to ISO 13485:2016
ISO 13485 is a international standard published by ISO for manufacturers of medical devices. It contains minimum requirements for a Quality management standard for medical device manufacturers. It is a extension of ISO 9001 tailored for medical devices manufacturers.
Who requires ISO 13485?
Generally, manufacturers and traders of medical devices require ISO 13485 based system. Some service provider for medical devices and software manufacturers also take ISO 13485 certification.
What are the Key requirements of ISO 13485?
While most of the requirements are same as ISO 9001. It has some very specific requirements:
- Post market surveillance or clinical followup
- clinical validation for medical devices
- risk analysis file for each medical device
- PEMS file for medical devices incorporating software
- A procedure for coordinating with authorities for advisory notices
Roadmap for Implementing ISO 13485 in Your Organization
ISO 13485:2016 standard sets out the requirements for a QMS (Quality Management System) for the development of medical devices. An organization, by implementing a QMS based on ISO 13485, exhibits its ability to deliver such medical devices that are in harmony with the regulatory and legal requirements and in line with the customer’s needs.
The prime focus of ISO 13485 QMS is to secure implementation and monitoring of high quality processes in the organization. The international standard strongly stresses on the organization’s commitment to the quality of their products, by way of:
- Effective process management and,
- A commitment to quality from all organizational levels
Meddevices Lifesciences Pvt Ltd has outlined an ISO 13485:2016 Implementation Roadmap to assist the organizations to implement an ISO 13485:2016 Quality Management System within their premises. The roadmap for implementation is given below in a flowchart.
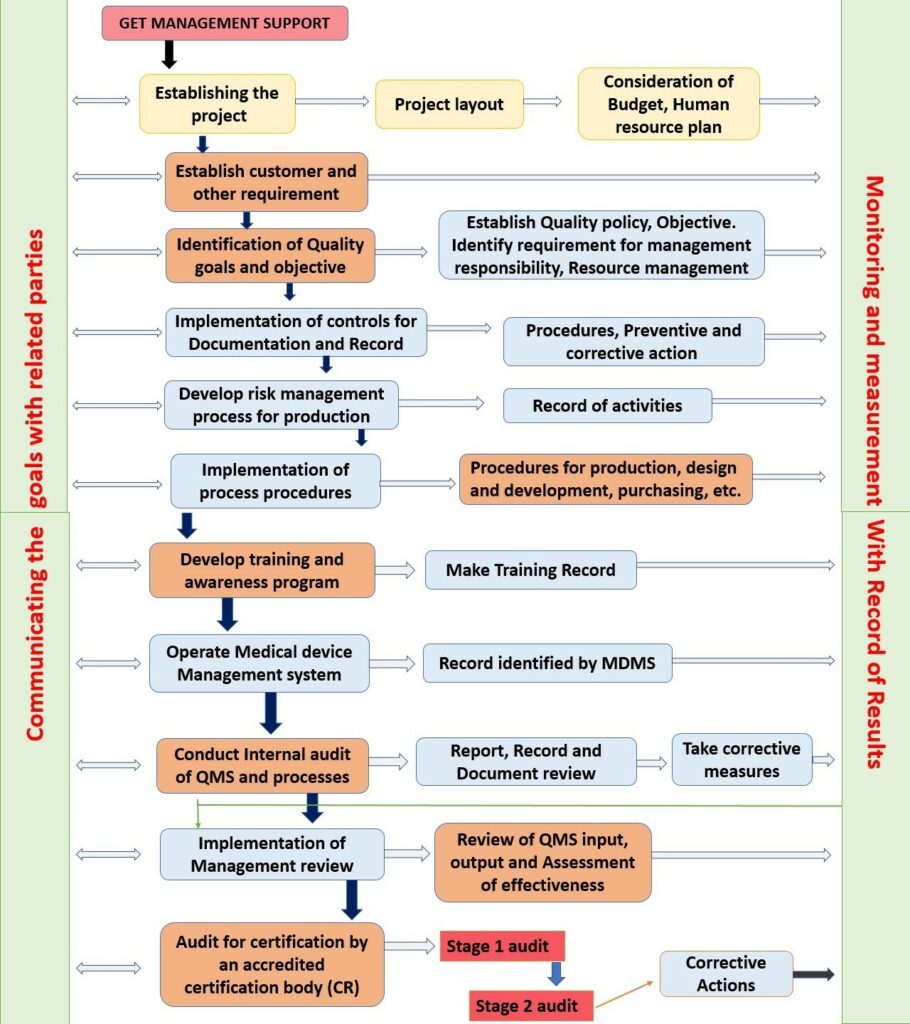
How Can I Get ISO 13485 Certificate?
ISO 13485 certificates can be obtained by a simple process, steps of which are described below in the flowchart:
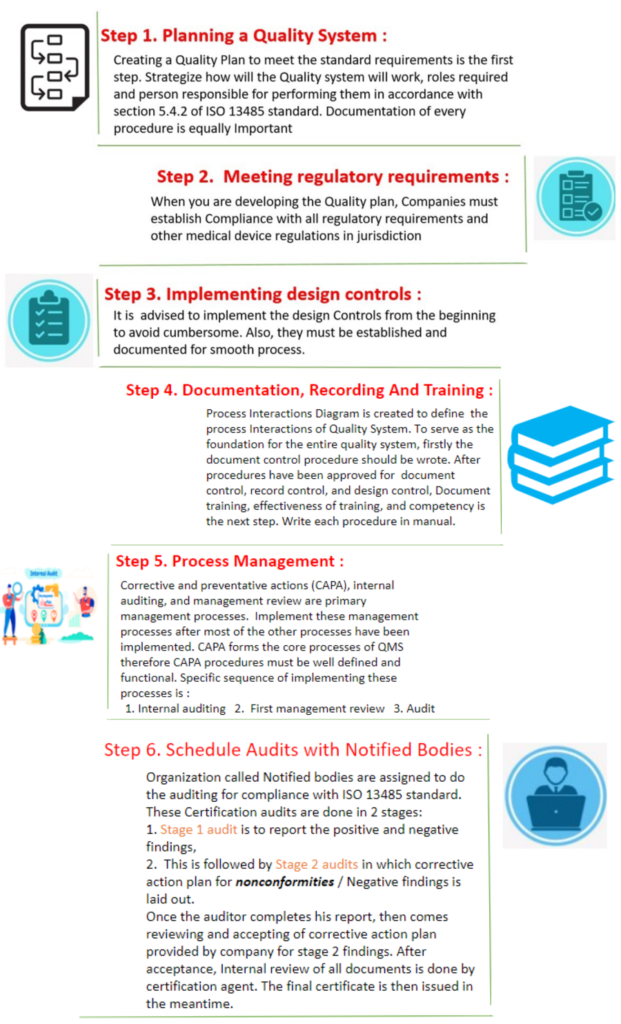
How Much Time Does it Take for Getting an ISO 13485 Certificate?
It generally takes 60 -120 days to get a ISO 13485 certificate
What are the Main Requirements of ISO 13485?
| Clause | Requirement |
| 4.1.1 | Roles |
| 4.1.2,4.1.3,4.1.4,4.1.5 | Sequence and interaction ( list of processes and outsourced processes and their risk level ) |
| 4.1.6 | Procedure for validation of software used in QMS |
| 4.2.1 and 5.3and 5.4.2and 5.4.3 | Quality Policy and objectives |
| 4.2.2 | Quality Manual |
| 4.2.3 | Medical Device Files to be asked from the Suppliers |
| 4.2.4 and 4.2.5 | Control of document and records procedure |
| 5.5.2 and 5.5.3 | Internal communication |
| 5.6.1, 5.6.2,5.6.3 | management review procedure |
| 5.5.1, and 6.2 | Human Resources ( Comeptency -ETSE, role risk , Awareness requirements) |
| 6.3 | Infrastructure( Building and Spaces ,equipment, softwares, supporting services) |
| 6.4 | Work environment and contamination control |
| 7.1 | Quality Plans for products |
| 7.2 | determination and review of requirements |
| 7.3 | Design and Development |
| 7.4 | Purchase |
| 7.5.1 | control of production and service provision |
| 7.5.2 | cleanliness of product |
| 7.5.3 | Installation activity |
| 7.5.4 | servicing activity |
| 7.5.5 | sterile requirments |
| 7.5.6 | validation of special processes |
| 7.5.7 | Validation of sterile barrier system |
| 7.5.8 | identification |
| 7.5.9 | traceability |
| 7.5.10 | customer property |
| 7.5.11 | preservation of product |
| 7.6 | Control of Monitoring and measuring equipment |
| 8.2.1 | Feedback |
| 8.2.2 | Complaint handling |
| 8.2.3 | Reporting to regulatory authorities |
| 8.2.4 | Internal Audit |
| 8.3 | Control of non conforming product (during production, before delivery , after delivery and rework) |
| 8.4 | Analysis of Data |
| 8.5.2 | corrective action |
| 8.5.3 | preventive action |
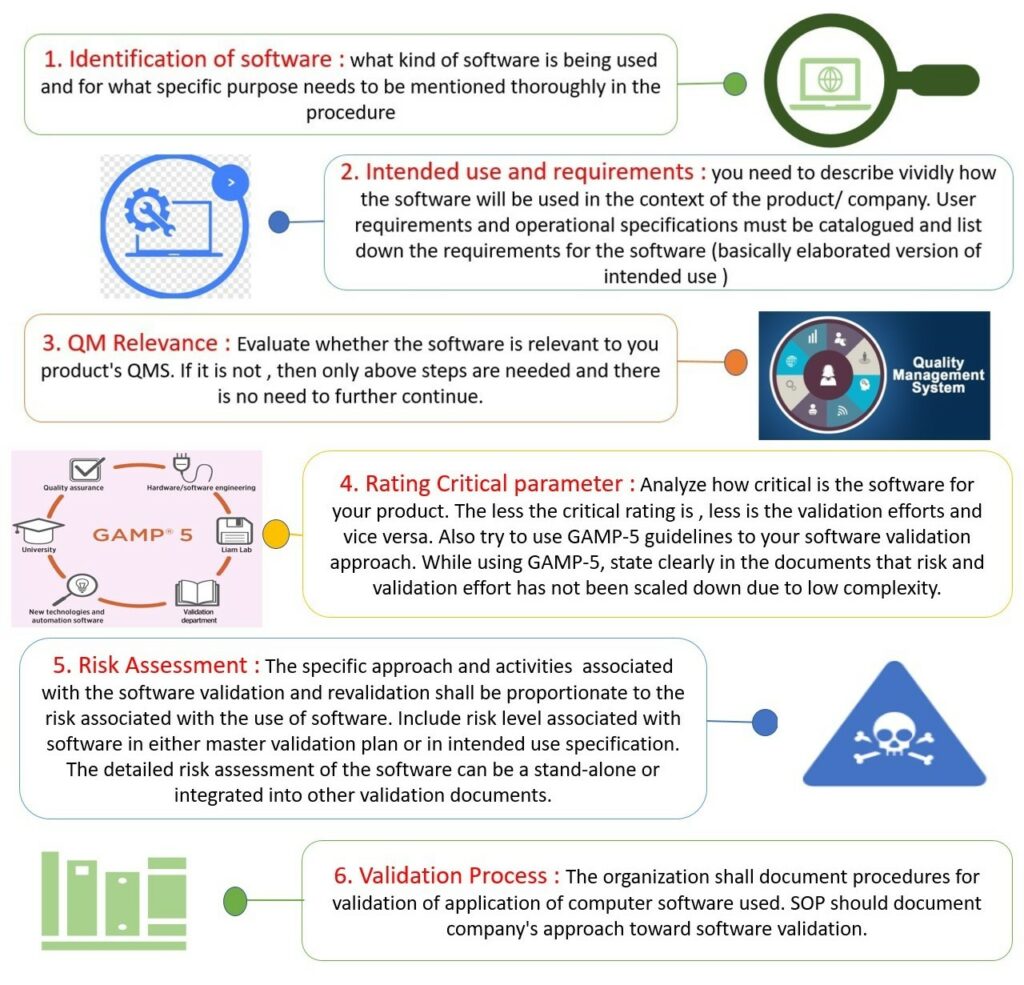
How to Validate Software According to ISO 13485?
When a company needs to demonstrate compliance with regulations and standards, the introduction of new software demands revalidation of the process to regularly check its correct configuration and operation after installation. This whole process is termed “software validation”.
Necessity to Validate Software
Software validation helps reduce risk and legal liability by providing evidence that the system is fit for purpose. Therefore, quality management software must be validated to control product quality and generate information for regulatory authorities when the system is used in good practice processes.
Plan of Action for Software Validation
ISO 13485:2016 Trainings
We at meddevices provide a range of training for ISO 13485 and related standards . Our trainings are done online with a maximum duration of 4 hours a day . We think that in a online course concentration levels of candidates drop after 4 hours.
We have developed the following types of courses for medical device manufacturers and traders
Medical Device Quality Assurance – A primer
| WHO IS IT FOR | Anyone who is new to medical devices and has no background of Quality Assurance or ISO 9001 |
| WHAT DOES IT COVER | We cover the following topics· Objectives of quality assurance and iso 13485· Basic of iso 13485 structure· Document control requirements· How to develop a procedure· Practice to Develop 3 simple procedure· Distinguishing records and procedures· How to control records |
| WHAT CAN YOU EXPECT | Hands on training based on activities and assignments to work onEach activity will be discussedSmall group of 3 people will be asked to work in a virtual room |
| TRAINING METHODOLOGY | ONLINEActivity and discussion basedShort assessments |
| TRAINING HOURS | Total 8 hours online for two consecutive days(4 hours each day) |
| PRICE | GBP 200 |
ISO 13485 IMPLEMENTER COURSE
| WHO IS IT FOR | Anyone who is either familiar with ISO 9001 or has attended our MDQA- Primer course |
| WHAT DOES IT COVER | We cover the following topics in ISO 13485 implementer courseDevelopment of 10 Key procedures for ISO 13485Review of these procedures with the requirements of ISO 13485Identify associated records with these procedureIdentify interactions between procedures |
| WHAT CAN YOU EXPECT | Templates of 10 Key procedures will be developed by you |
| TRAINING METHODOLOGY | ONLINEActivity and discussion basedShort assessments |
| TRAINING HOURS | Total 8 hours online for two consecutive days(4 hours each day) |
| PRICE | GBP 200 |
