After manufacturing the medical device, there is one very important step that is considered to be a part of the manufacturing process, which many medical device manufacturers have to take, before the device reaches in the hands of the end users around the world.
STERILIZATION!!
Poorly sterilized or unsterilized devices pose a serious threat to the health of its users. Though there are a number of ways to sterilize a medical device, the decision to use one method over the other is based on various factors.
One of the most common methods of sterilization is to use ethylene oxide (EO) gas.
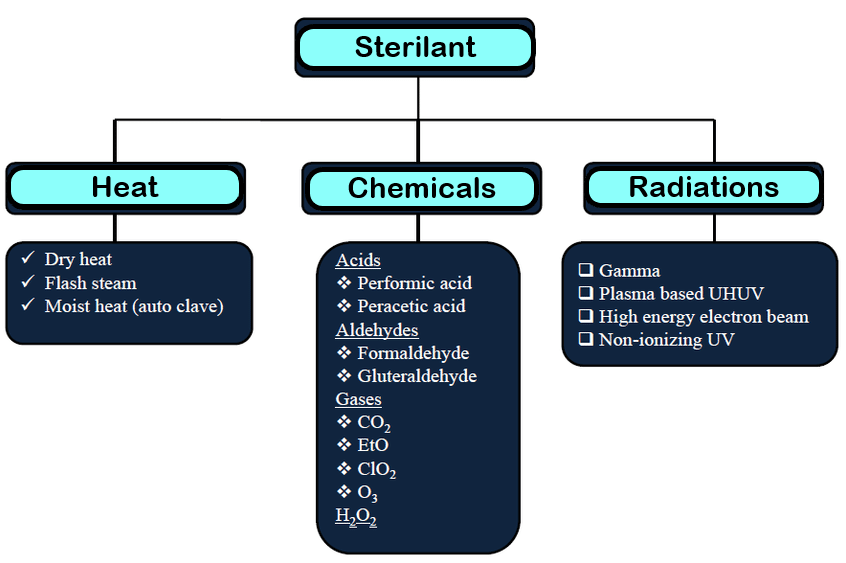
Though ethylene oxide is an excellent sterilant, it requires a close attention to detail for safe and effective use, as EO is highly flammable and carcinogenic. One of the major reasons for EO being a popular sterilant is its ability to infiltrate plastic packaging, and sterilize the device(s) inside. Needless to say, it is impossible to verify that each device has been properly sterilized without opening the package and compromising the sterility. ISO 11135 lays guidelines for the manufacturers to validate this sterilization process.
Once the validation is completed, the device can be confidently said to be sterilised.
EO Sterilization Process – As per ISO 11135
ISO 11135 divides the EO sterilization process into 3 stages:
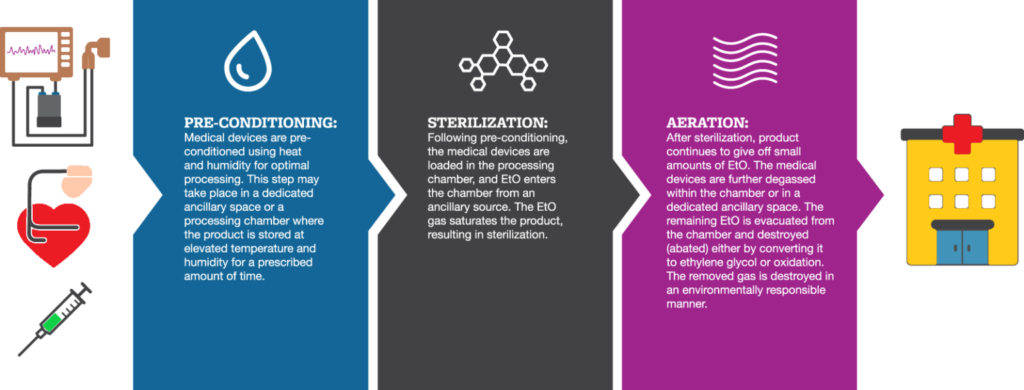
Stage 1: Preconditioning
In this step, the chamber in which the products are to be sterilized is prepared. It is preconditioned for humidity and temperature. Preconditioning has 2 main purposes:
- It conditions the packaging material so that EO can penetrate easily.
- The humidity conditions the cellular walls of any micro-organisms so that they are less resistant to the gas.
Stage 2: Sterilization
Since EO is flammable at around 10% oxygen concentration, the air in the chamber is first removed using a vacuum. After removing the air, the EO is inserted into the chamber and the sterilization process begins.
Stage 3: Aeration
This is the final step of the sterilization process. In this, the EO is removed from the chamber. The removal is usually done by flushing the chamber with an inert gas (like nitrogen) or steam. After that, the product is placed in an aeration chamber that circulates hot air, to eliminate any residual EO that might otherwise be absorbed in the product.
Validation Guide for EO Sterilization – As per ISO 11135
Once the sterilization process is completed, the manufacturer still needs evidence that the bio-burden present on the product has been destroyed in the sterilization process. The validation process can be done by using ISO 11135 as your guide.
Process Validation consists of 3 stages.

Stage 1: Installation Qualification (IQ)
It ensures that the equipment required for the sterilization process is installed correctly.
Stage 2: Operational Qualification (OQ)
It ensures that the equipment installed for carrying out the sterilization process is operating correctly.
Stage 3: Performance Qualification (PQ)
This is the final and most important stage. PQ proves that the process is able to consistently sterilize your products under real-world conditions. This stage is further divided into 2 steps:
- Micro-Biological Performance Qualification
- Physical Performance Qualification
Micro-Biological Performance Qualification (MPQ)
During MPQ, the manufacturer decides the minimum process parameters so as to ensure that the product’s micro-organisms are destroyed. For this, a Biological Indicator (BI), which is generally a strip covered in at least a million bacteria with resistance to ethylene oxide, is used in the product to simulate contamination.
‘Overkill’ method is the most commonly used method of MPQ. It constitutes running a half-cycle i.e. half the standard time the product is in the sterilization chamber, and then revealing that the half-cycle is sufficient enough to destroy the BI.
If 3 consecutive half-cycles destroy the BI, it can be assumed that the full cycle the manufacturer plans on running will be ‘overkill’.
Physical Performance Qualification (PPQ)
Physical Performance Qualification consists of at least 3 full cycles of sterilization, and must demonstrate that the process can be duplicated and that the product performs as intended even after sterilization.
Though process validation is necessary to ensure the sterilization and safety of your medical device, documenting your conformity with standards like ISO 11135:2014 and ensuring that it is readily available is also essential.
