A Free Sales Certificate is a pre-requisite for every manufacturer of medical devices who is aspiring to do business in open market. While exporting your medical device, the Certificate of Free Sale (CFS), is an ‘essential’ for the manufacturer to ensure that the device is already available in the market of the exporting country.
Health Ministry of many countries, and regulators around the world, demand certificate of free sales from the manufacturers, to ensure that their products are approved for sales outside the origin country. An FSC, also termed as ‘Certificate of Export’ or ‘Certificate of Free Sales’, testifies that the device is safe to be used in the country to which it is being exported. For the manufacturers of the medical devices manufactured in European Union, it is essential to ensure that the FSC endorses that the exporting medical device is CE Marked.
Purpose of FSC
Securing an FSC is crucial for many reasons for the manufacturers who are going international with their medical products. For EU based manufacturer of medical devices, FSC is proved to be very crucial to capitalize the Middle Eastern healthcare sector which always remains in a great demand of medical instruments which are already in use in the territory of the EU. The Certificate of Free Sales can be used as a proof for:
- Verification of Product: Importing countries maintain a strict level of safety in their healthcare sector. Therefore, they don’t allow the use of pharmaceutical products, medical devices and other critical products from a manufacturer that cannot furnish a proof that the medical product being exported is authorized to be used in the country where its operations are based.
For this purpose, CFS is a valuable piece of information, where the associated regulatory body vouches for the importing manufacturer that the certified medical device is an authorized healthcare product in the county where it is manufactured.
- Verification of Manufacturer: Though the verification that the manufacturer is an authorized business entity can be done through several ways, CFS is a definitive proof that the manufacturer contains a valid license. Authentication of manufacturer’s license automatically validates that the business is complying with the guidelines needed for CE marking.
- Verification of Good Manufacturing Practice Compliance (GMP): An FSC is also a proof of the fact that the device in question complies with GMP guidelines of International Society of Pharmaceutical Engineering. In addition to CE marking, this is another important certification which further endorses the trustworthiness of any medical product. The fact that makes FSC Certificate more important is that it is, in essence, a guarantee issued to the importing country by the exporting country about the authenticity of the manufacturer and its product.
Who Issues FSC?
All European countries are eligible to issue European Free Sales Certificate (FSC). By protocol, it is to be issued by the member state where the manufacturer wants to sell their medical device. An Authorized Representative submits an application on the behalf of the manufacturer to an European Competent Authority for issue of the FSC.
Steps to Apply for a Free Sales Certificate
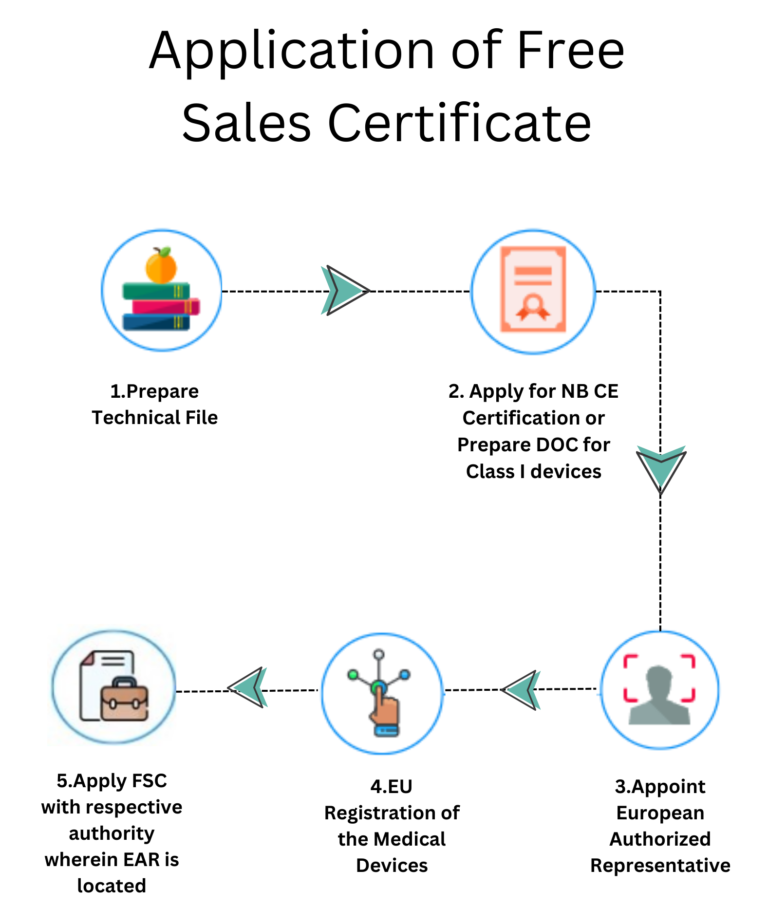
Which Medical Devices are Eligible for FSC?
Medical devices that possess CE mark and the devices that are marketed inside the European Union are eligible to get the Certificate of Free Sales.
Who can Apply for FSC?
Any manufacturer or his Authorized Representative who wants to sell his medical device in Europe and comply with EU Regulations for medical devices is eligible to apply.
Duration of Time
The duration from filing the application to the issuance of the certificate is 4-5 weeks. It takes additional 10 to 14 days if the FSC needs apostille and legalization.
Documentation Requirement
Generally, the European Authority, to issue a Free Sales Certificate for medical device, requires the following documentation:
- Class I: Declaration of Conformity + Technical Documentation.
- Class Is, Im, IIa, IIb: Declaration of Conformity + CE Certificate.
- Class III: Declaration of Conformity + CE Certificate + Design Examination Certification (DEC).
Legalization and Apostille of FSC
Apostille or legalization of FSC is important because a legalized Free Sales Certificate acts as a proof of compliance with the Regulatory System. It gives an assurance that the medical device mentioned on the certificate is evaluated for safety and efficacy and can be freely sold in Europe. Legalization or apostille of a Certificate of Free Sales proves the genuineness and accuracy of the certificate. With the apostille stamp, the certificate becomes recognised by other country’s External Ministry.
There are three institutions that have the authority to legalize the FSC.

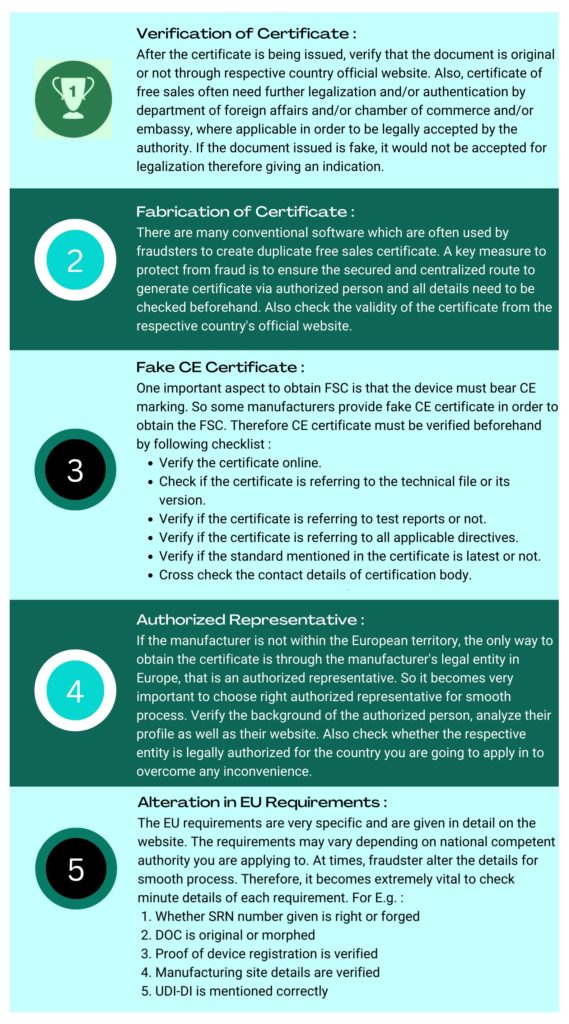
Fake Free Sales Certificate
Free Sales Certificate is a critical document for medical device trade in European Union countries. It is a must have document to trade in medical device inside European Union. The process is quite simple but not everything is as it seems. It is very common practice to make fake or alter the existing certificate of the manufacturer for smooth processing. Therefore, it becomes a necessity to distinguish between fake and original Free Sales Certificate and help protect those who are at risk of fraud.
The 5 core aspects to protect against fraud of fake certificate are as follows:
What We Provide?
For the manufacturers of medical device striving to go global with their medical devices, we provide to them:
Free Sales Certificate from MHRA UK:
We provide FSC from MHRA for medical Devices from our company ‘Med Devices Lifesciences Ltd’ based in UK. This Free sales certificate is well accepted in Egypt/Middle East and other countries.

Free Sales Certificate from Europe:
We provide FSC from Netherlands for medical Devices from our company ‘Med Devices Lifesciences BV’. This Free Sales Certificate is well accepted in Egypt/Middle East and other countries.

We also provide the necessary testing required for CE Marking and other relevant certifications.
